
Case study – R&D improvement study of dental product processing with instrumented micro-pilot technique
Sector : Pharmaceutics
Type : Group
Department : R&D / Industrialisation
The Client is facing issues with texture variabilites in industrialisation tests for dental product process while FDA certification in progress. RHEONIS is here to draw out the causes and influencing factors for such variabilities and recommend improvements of the process and any related aspect.
Combining industrial expertise and R&D special techniques
In such study, RHEONIS provides its industrial process expertise, its knowledge of DoE analysis and its special techniques for R&D with instrumented micro-pilots.
During this phase-segmented collaboration, we performed the following tasks :
- On site industrial process diagnosis during production
- Industrial Phenomenology analysis with respect to the DoE and its results
- Synthesis and recommendations for R&D study
- Design of special mixing tool for instrumented mixing micro-pilot on rheometry platform
- R&D study and conclusions on variability causes and influencing factors
- Synthesis and operational recommendations


Building an appropriate Design-Space and improving process
For a complex formula with dozen of ingredients, industrialisation trials are often constrained with economical limits.
Instrumented micro-pilot technique allows a fast, advanced and low-matter-consumption of ingredients impacts and processing (ingredient introduction and mixing speed/duration) in real-time sequences.
Various parameters can be monitored, so that critical steps can be clearly identified and their impact on product texture quantified.
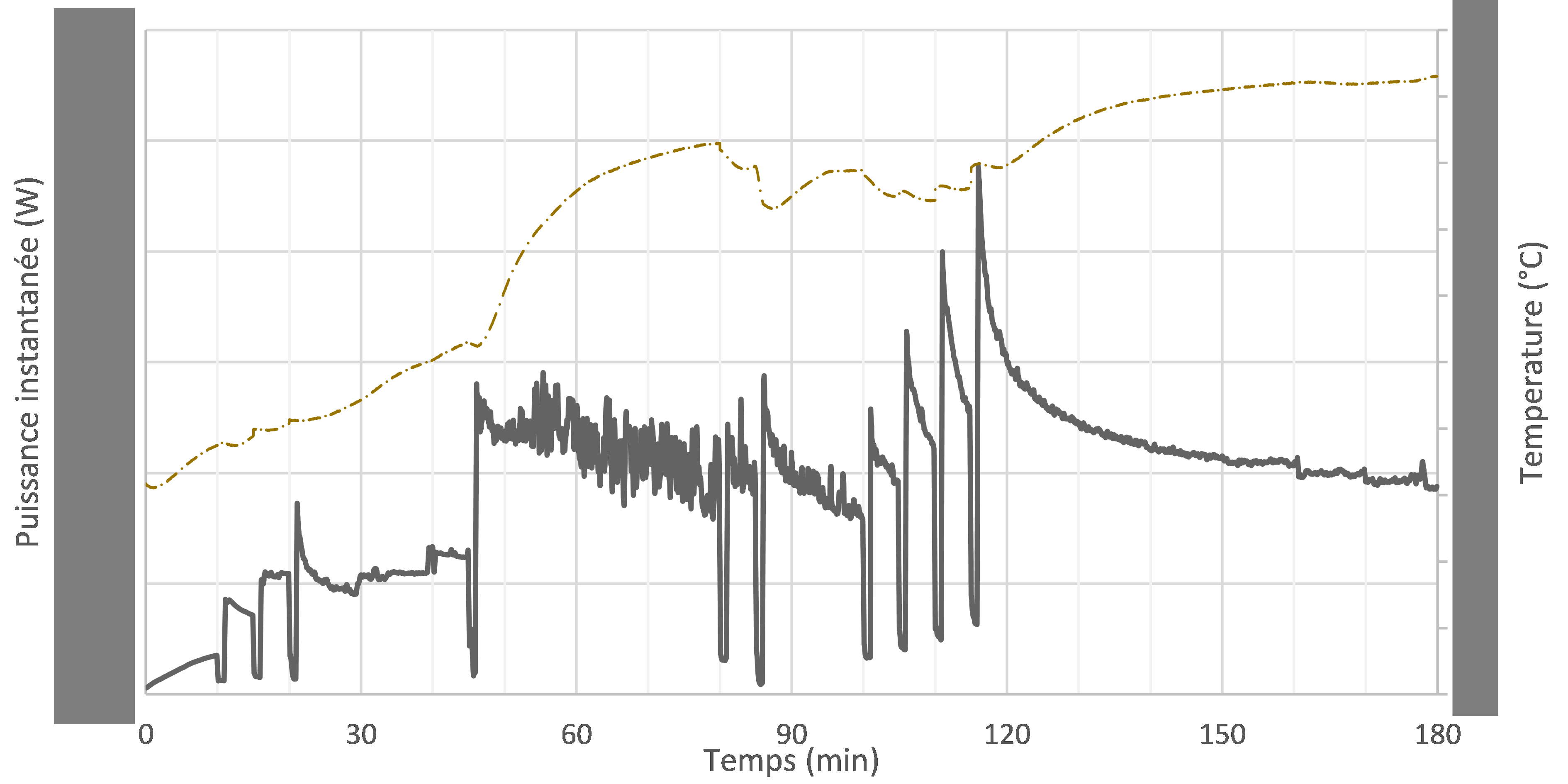
Optimised processing conditions can thus be determined and effetcs such as hygrometry can be identified, allowing the building of an accurate design-space for parameters of interest.
Strengths of RHEONIS for pharmaceutical challenges
Quality By Design-oriented expertise
Low-matter consumption for product/process studies
Focus on phenomena and on-site diagnosis for DoE size reduction
Pragmatical approach for operational recommendations
Any question regarding projects and issues in pharmaceutical field ? Feel free to contact us.
Last Updated on 20 octobre 2021 by Vincent Billot
